Digital health has experienced significant changes over the past decade in terms of technology, infrastructure, and adoption. The surge in telemedicine, remote patient monitoring, and artificial intelligence helped improve healthcare delivery to be more efficient, cost effective, equitable, and accessible to patients where they are.
The COVID-19 pandemic has certainly accelerated this transformation. The sophistication of mHealth technologies (such as wearables) and their integration into people’s daily lives opened doors to the wellness and lifestyle industry, bringing us closer to a more holistic patient care, where prevention is as important as treatment. However, truly remarkable is the quiet transformation brought by Digital Therapeutics. Digital is no longer only a support to clinical decision-making and delivery. It has become a viable form of healthcare intervention with these Digital Therapeutics, at the same level of clinical impact as pharmaceuticals or medical devices. In the last few years, studies have been published to demonstrate their effectiveness in chronic disease management, mental health, personalised care, and diagnosis.
Related: Create a future health system paradigm to reform the industry
Since 2017, digital therapeutics has had the fastest growth of any healthtech segment, increasing by eight-fold in the last five years with an estimated global market value of US$28.7 billion in 2030. With the market projected to grow substantially, digital therapeutics holds immense potential for transforming healthcare systems to help cope with the rising demands of our society, both present and future.
Status of digital health adoption in the Asian market
According to Galen Growth, 27 per cent of digital health ventures were funded in Asia Pacific in 2022, making it the second largest ecosystem globally. Countries like China, South Korea, and Singapore are emerging as innovation hubs, fostering local start-ups. Research and online marketplace clusters captured 49 per cent of the total funds invested. The integration of e-commerce and healthtech has gained momentum, offering convenient access to medication and healthcare services, such as the internet hospitals in China. Telemedicine has been widely adopted and continues even after the COVID-19 pandemic. However, Asia-Pacific is a diverse and complex region. Variations in healthcare systems, legal and regulatory policies, economic and cultural disparities can be significant across different Asian countries. The contrasting and fragmented healthcare infrastructures are compounded by inconsistent levels of connectivity and digital literacy, particularly in rural areas. These challenges can limit the effectiveness and adoption of digital health solutions.
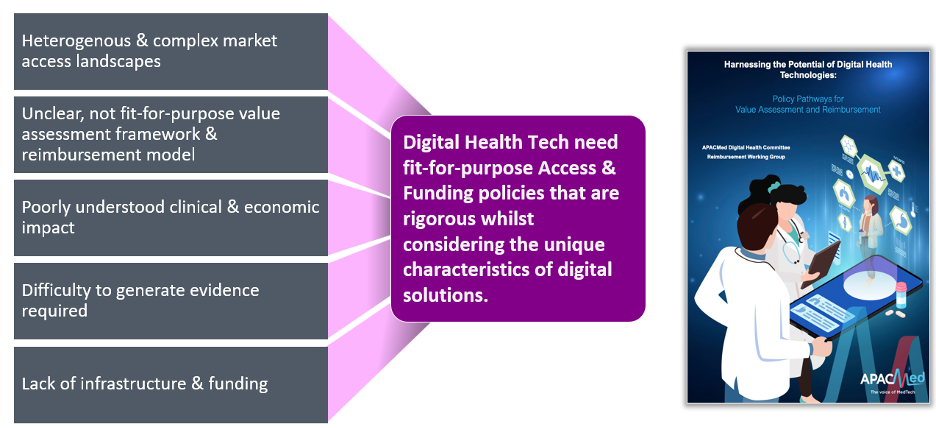
Moreover, economic disparities necessitate well-designed business models to make digital health solutions affordable for sustainable implementation. However, there is currently no specific evaluation framework in the region to appropriately recognise the full value of these innovations, resulting in fragmented reimbursement coverage and under funding. This lack of evaluation framework tailored to digital health further limit and delay patient access in Asia-Pacific. The reimbursement pathways and evidence requirements are often unclear, and usually not suitable for many digital health technologies. Therefore, there is a need to better craft access strategies for digital health technologies to address these barriers.
Digital health tech vs. traditional health products
The rapid, incremental, iterative nature of digital health technologies make them stand out from traditional drugs or medical devices, making their evaluation more challenging. However, without an appropriate assessment, their value would be under-appreciated. This subsequently would have a significant impact on the willingness-to-pay, purchasing, and funding/reimbursement decisions. Unlike traditional drugs or devices, which remain relatively consistent while in use, digital health technologies are frequently updated. This dynamic nature can make less applicable to the traditional methods of evaluation designed for static products, such as randomised double-blinded clinical trials.
Moreover, the causal effect between the intervention and the outcome of interest is not linear with digital health technologies, as with traditional health products. The effectiveness of these technologies hinges on product usage and user engagement. Variability in how users interact with these technologies can significantly impact their performance and outcome, a factor that traditional evaluation methods for drugs or devices might not fully account for.
Related: AI-powered tools drive diagnostic precision in anatomical pathology
Lastly, digital health technologies integration into existing healthcare systems often requires a disruption of workflow and organisational change. Their health economic benefits can vary widely depending on the specifics of the healthcare system. Therefore, digital health technologies would need broader and more comprehensive, fit-for-purpose health technology assessment frameworks.
Understanding their unique attributes is vital for developing evaluation models and processes that can accurately measure their overall value to different stakeholders in healthcare (patients, practitioners, providers, policymakers and payers).
Drivers for access of digital health tech in Asia
Despite its many challenges, Asia offers vast potential for digital health. Governments are investing in digital health infrastructure, such as India’s Ayushman Bharat Digital Mission. They should continue this positive trend by implementing supportive policies, providing funding for digital health initiatives, and creating a favourable regulatory and access environment. Until recently, policymakers in the region have tended to rely on existing medical device policies for Digital Health. However, the unique nature and characteristics of digital health call for revised policies that are fit-for-purpose. These innovations introduce a new paradigm for healthcare delivery, where much is still to be created and no one has all the answers. Thus, it is not only incumbent on governments to drive appropriate access and adoption approaches. It falls on all players in the ecosystem to collaborate. Manufacturers should work closely with other stakeholders to ensure their innovative digital solutions meet the needs of diverse populations and bring value.
Healthcare providers and professionals can ensure effective integration into the healthcare delivery system. They can help articulate and educate patients and policymakers about the benefits of digital health and strengthen trust. Digital offers the possibility for patients to take ownership of their health. Hence, they need to be actively engaged in the development, implementation, and update of digital health solutions throughout the product lifecycle.
Collectively, we can improve the effectiveness of Digital Health Technologies for the care quality that everyone deserves, and simultaneously accelerate the time-to-market for innovations that will have wider socioeconomic benefits.

Anh Bourcet (Nguyen), PhD, is the Strategic Advisor at the Asia Pacific Medical Technology Association (APACMed). A seasoned global market access leader, she will present her topic on “Broadening Patient Access to Digital Health” at the Digital Health conference, and “Digital Health – Reimbursement Pathways” under the Doing Business in Thailand segment on August 16 and 18, respectively, at Asia Health 2023.
Learn more about Medlab Asia and Asia Health and click here to register for the event.
This article appears in Omnia Health magazine. Read the full issue online today.


