A number of countries have now started to report that the rate of COVID-19 cases has started to decline. However, the progress that is being watched even more closely is the development of a Coronavirus vaccine.
It normally takes years to develop, approve and produce an effective vaccine. But due to the need of the hour, there are currently over 70 COVID-19 vaccines in development worldwide, according to the World Health Organization (WHO).
Creating a safe vaccine would provide some protection by training people's immune systems to fight the virus so they don’t get infected. It would, therefore, allow lockdowns to be lifted and social distancing rules to be relaxed. Below we take a look at the progress of a few developments:
Oxford COVID-19 vaccine begins human trial stage
University of Oxford researchers have begun testing a COVID-19 vaccine in human volunteers. Around 1,110 people will take part in the trial, half receiving the vaccine and the other half (the control group) receiving a widely available meningitis vaccine. They will reportedly have a million doses ready for use by September.
The researchers started screening healthy volunteers (aged 18-55) in March for their upcoming ChAdOx1 nCoV-19 vaccine trial. The vaccine is based on an adenovirus vaccine vector and the SARS-CoV-2 spike protein and has been produced in Oxford, UK.
It aims to assess whether healthy people can be protected from COVID-19 with this new vaccine called ChAdOx1 nCoV-19. It will also provide information on the safety aspects of the vaccine and its ability to generate good immune responses against the virus.
ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weakened version of a common cold virus (adenovirus) that causes infections in chimpanzees, that has been genetically changed so that it is impossible for it to grow in humans.
Genetic material has been added to the ChAdOx1 construct, that is used to make proteins from the COVID-19 virus (SARS-CoV-2) called Spike glycoprotein (S). This protein is usually found on the surface of SARS-CoV-2 and plays an essential role in the infection pathway of the SARS-CoV-2 virus. The SARS-CoV-2 coronavirus uses its spike protein to bind to ACE2 receptors on human cells to gain entry to the cells and cause an infection.
By vaccinating with ChAdOx1 nCoV-19, the hope is to make the body recognise and develop an immune response to the Spike protein that will help stop the SARS-CoV-2 virus from entering human cells and therefore prevent infection.
Vaccines made from the ChAdOx1 virus have been given to more than 320 people to date and have been shown to be safe and well-tolerated, although they can cause temporary side effects, such as a temperature, headache or sore arm.
The University of Oxford recently also announced an agreement with the UK-based global biopharmaceutical company AstraZeneca for the further development, large-scale manufacture and potential distribution of the COVID-19 vaccine being trialled.
Pfizer and BioNTech to co-develop vaccine
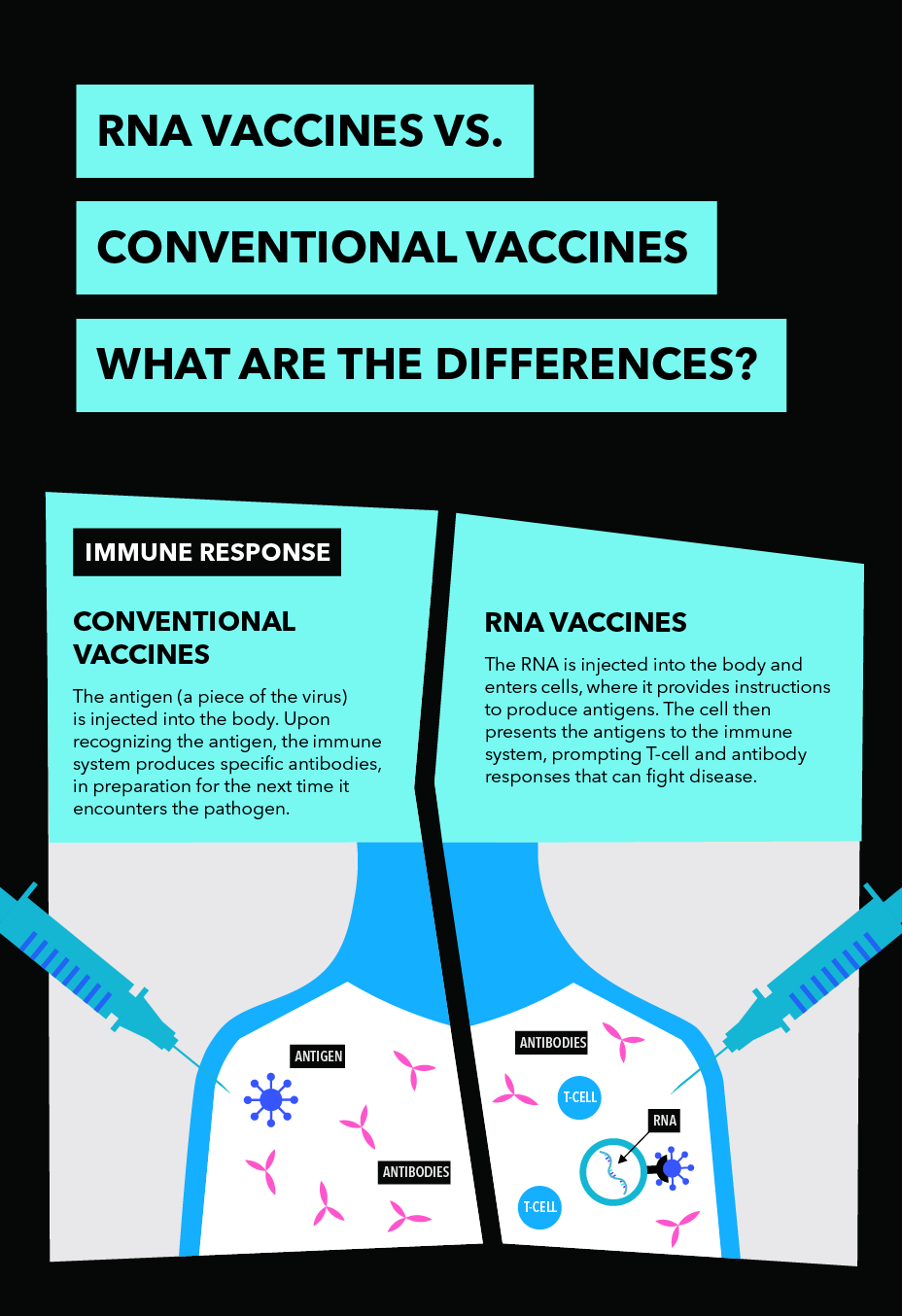
Pfizer Inc. and BioNTech SE announced that the companies have agreed to a letter of intent regarding the co-development and distribution (excluding China) of a potential mRNA-based coronavirus vaccine aimed at preventing COVID-19 infection.
The collaboration aims to accelerate the development of BioNTech’s potential first-in-class COVID-19 mRNA vaccine programme, BNT162, which was expected to enter clinical testing by the end of April. The rapid advancement of this collaboration builds on the research and development collaboration into which Pfizer and BioNTech entered in 2018 to develop mRNA-based vaccines for prevention of influenza.
Sanofi and GSK collaborate
Sanofi and GSK recently announced that they have signed a letter of intent to enter into a collaboration to develop an adjuvanted vaccine for COVID-19, using innovative technology from both companies, to help address the ongoing pandemic.
Sanofi will contribute its S-protein COVID-19 antigen, which is based on recombinant DNA technology. This technology has produced an exact genetic match to proteins found on the surface of the virus, and the DNA sequence encoding this antigen has been combined into the DNA of the baculovirus expression platform, the basis of Sanofi’s licensed recombinant influenza product in the U.S.
GSK will contribute its proven pandemic adjuvant technology to the collaboration. The use of an adjuvant can be of particular importance in a pandemic situation since it may reduce the amount of vaccine protein required per dose, allowing more vaccine doses to be produced and therefore contributing to protecting more people.
The combination of a protein-based antigen together with an adjuvant is well-established and used in a number of vaccines available today. An adjuvant is added to some vaccines to enhance the immune response and has been shown to create a stronger and longer-lasting immunity against infections than the vaccine alone. It can also improve the likelihood of delivering an effective vaccine that can be manufactured at scale.
The companies plan to initiate phase I clinical trials in the second half of 2020 and, if successful and subject to regulatory considerations, aim to complete the development required for availability by the second half of 2021.
Moderna announces Phase 2 Study of mRNA Vaccine
Moderna, Inc., a clinical-stage biotechnology company, recently announced that it has submitted an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for the company’s mRNA vaccine candidate (mRNA-1273) against the novel coronavirus (SARS-CoV-2) to evaluate mRNA-1273 in Phase 2 and late-stage studies if supported by safety data from the Phase 1 study led by the National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health.
Moderna has received initial feedback from the FDA on the design of the planned Phase 2 study, which is expected to begin in the second quarter of 2020. This study will evaluate the safety, reactogenicity and immunogenicity of two vaccinations of mRNA-1273 given 28 days apart. Each subject will be assigned to receive placebo, a 50 μg or a 250 μg dose at both vaccinations. The company intends to enrol 600 healthy participants across two cohorts of adults ages 18-55 years (n=300) and older adults ages 55 years and above (n=300). Participants will be followed through 12 months after the second vaccination.
Subject to data from Phase 1 and Phase 2 studies and discussions with regulators, a Phase 3 study could begin in the fall.
Johnson & Johnson to initiate human clinical studies by September
Johnson & Johnson recently announced the selection of a lead COVID-19 vaccine candidate from constructs it has been working on since January 2020. The company has been working with Biomedical Advanced Research and Development Authority (BARDA) and has rapidly scaled manufacturing capacity with the goal of providing a global supply of more than one billion doses of a vaccine. The company expects to initiate human clinical studies of its lead vaccine candidate at the latest by September and anticipates the first batches of a COVID-19 vaccine could be available for emergency use authorisation in early 2021.
BARDA, which is part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services, and Johnson & Johnson together have committed more than US$1 billion of investment to co-fund vaccine research, development, and clinical testing.

